
The journalists at BuzzFeed News are proud to bring you trustworthy and relevant reporting about the coronavirus. To help keep this news free, become a member and sign up for our newsletter, Incoming.
The FDA authorized Moderna’s COVID-19 vaccine on Friday, clearing the way for a second weapon in the historic effort to immunize the country against a deadly virus that has killed more than 300,000 Americans.
The decision followed an overwhelming endorsement from an FDA advisory panel on Thursday, which voted 20–0, with one abstention, in its favor. The two-dose vaccine was found in a late-stage clinical trial to prevent 94.1% of symptomatic coronavirus cases, when compared to people who got placebo shots, and caused no serious reactions. Those characteristics make it similar to Pfizer-BioNTech’s vaccine, which started being administered across the US this week.
Moderna’s vaccine appears to have some key advantages over Pfizer’s, making it a possibly even more powerful tool. It can be much more easily transported and stored, and data suggests that it may be better at preventing severe disease.
"The evidence that has been studied in great deal on this vaccine highly outweighs any of the issues that we’ve seen," Hayley Gans, a pediatrician at Stanford University, said at the panel meeting on Thursday. "It really supports us being able to, with the pandemic in our background, really move forward and finally provide a safe and effective way to get to herd immunity."
About 2.9 million doses of Pfizer’s vaccine were shipped out across the country this week, and another 2 million are slated to be delivered next week. Nearly 6 million doses of Moderna’s newly authorized vaccine are scheduled to go out next week, according to federal officials with Operation Warp Speed, the federal government’s private-public investment effort to develop coronavirus therapeutics and vaccines. They would be the first set of a total of 100 million doses planned to be delivered by the first quarter of 2021, soon to be followed by an additional 100 million doses recently secured by the federal government.
The nation’s 21 million healthcare workers and 3 million long-term care facility residents should be first in line, according to CDC recommendations, though states have the ultimate say on who gets priority.
Now that two vaccines will be available under an “emergency use authorization” — which is based on less data than a full approval — the challenge will be to convince people to take them. This week, the Trump administration finally began a long-delayed rollout of a $250 million campaign to educate people about COVID-19 vaccines.
“We've been heartened to see that Americans' vaccine confidence is rising substantially,” Alex Azar, head of the Department of Health and Human Services, said at a Wednesday press conference. He cited a Kaiser Family Foundation poll in which 71% of respondents now say they would get a vaccine, up from 63% in November. According to other surveys and anecdotes, many, though not all, health care workers are enthusiastic about getting vaccinated.
Ahead of the vote at Thursday’s FDA advisory panel meeting, FDA scientists and Moderna representatives presented efficacy and safety data about the vaccine. Here’s what you need to know from the numbers and the questions the experts asked:
1. This is another highly effective vaccine.
Like Pfizer’s vaccine, Moderna’s two doses — given 28 days apart — are made from a genetic molecule called messenger RNA, or mRNA, which has been studied for other medical purposes, including in human clinical trials, for more than three decades. Pfizer and Moderna’s COVID-19 vaccines will be the first mRNA shots authorized by the FDA.
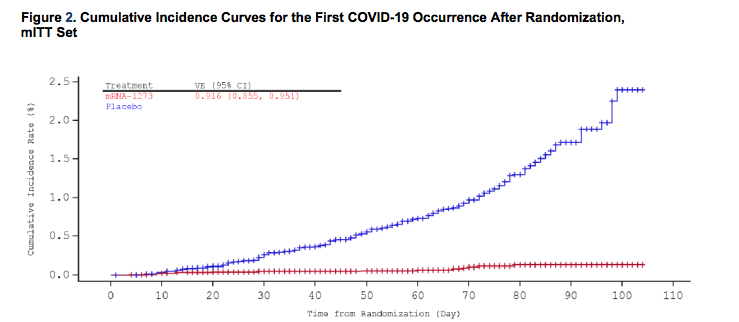
Moderna’s late-stage clinical trial now involves 30,000 participants aged 18 and up, half of whom got the vaccine and half who got the placebo. According to the company’s most recent data analysis in late November, which was released and confirmed by the FDA on Tuesday, the vaccine was 94.1% effective at preventing COVID-19: 185 people in the placebo group got sick, compared with just 11 in the vaccine group. That rate was similar across age groups, genders, racial and ethnic groups, and people with conditions that can heighten the severity of COVID-19.
That extremely high efficacy rate surprised many scientists when the preliminary results were first announced by press release in November. For reference, the FDA’s minimum threshold for efficacy in a COVID-19 vaccine is 50%.
“The efficacy data remain stunning,” David Benkeser, a biostatistician at Emory University who is not on the FDA’s advisory panel, said by email.
How was a case defined? In addition to testing positive, a person had to have at least two symptoms affecting the whole body (such as fever and chills) as well as at least one respiratory symptom (such as a cough or difficulty breathing). That was a stricter definition of a mild case than the one used by Pfizer, which defined an infection as having just one symptom plus a positive lab result.
It’s worth noting that study participants still wore masks and followed distancing guidelines where they lived. The vaccine wasn’t a magic bullet.
2. Some data suggests Moderna’s vaccine may be able to prevent asymptomatic cases, too.

An open question has been whether the vaccines tested so far can also prevent people from getting infected and passing the infection on to others, versus just getting sick enough to have symptoms.
Importantly, the new data show that Moderna’s vaccine might also help prevent asymptomatic infections — that is, cases in which people test positive without having symptoms — which are believed to make up as much as 20% to 30% of all COVID-19 cases, and to be a significant driver of transmission because people spread the virus to others without realizing they’re sick.
This data was not submitted by Moderna to the FDA in its application for an emergency use authorization, but was collected after the fact and disclosed this week. What it showed was that in the time between receiving the first and second doses, 14 people in the vaccine group tested positive with no symptoms, while 38 people in the placebo group did. This roughly two-thirds difference, according to Moderna, suggests that “some asymptomatic infections start to be prevented after the first dose.”
Benkeser called the data on asymptomatic prevention “fascinating and intriguing,” but “probably too preliminary” to put much stock into at this point. At the FDA panel meeting, some experts suggested nasal swab testing of any study volunteers who choose to drop out of the trial to receive a vaccine — many participants are health care workers — to gather more data on these asymptomatic infection rates.
Deepta Bhattacharya, an immunologist at the University of Arizona who is not part of the FDA’s advisory panel, said by email that he considered the asymptomatic case reports “the most exciting preliminary data,” adding, “There are not many numbers here, but the difference is pretty big and looks statistically meaningful.”
“The upshot is that if it is preventing infections outright, then it will have a big impact on lowering community transmission,” Bhattacharya said. He added that even those who got vaccinated who do get infected will likely have lower amounts of virus in their systems, which makes them less likely to pass the virus on to others.
During a press conference Friday, Peter Marks, director of the FDA’s Center for Biologics Evaluation and Research, said the NIH was considering initiating a study to get clearer data about this question. It may be possible to do one in groups of people who won’t be eligible for a vaccine in the immediate future, such as college students. “That is a critical thing to get answered, whether these vaccines can prevent asymptomatic transmission,” he said.
3. The vaccine also appears to be very safe, but you’ll likely have a sore arm.
Many people who get vaccinated will get a headache, fatigue, and arm soreness. Some may feel feverish. These reactions go away in a day or so. They're normal and indicate that the vaccine is working.
The safety data, too, was encouraging, with FDA staff reporting “no specific safety concerns” about the vaccine. That assessment is based on data from more than 15,000 participants who received the shots, half of whom were followed for two months after their second dose. Side effects were common and typical of the mild to moderate reactions associated with vaccines in general: soreness, fatigue, headaches, muscle pain, joint pain, and chills. There wasn’t enough data to draw conclusions about how safe the vaccine was for people who were pregnant or had immunocompromised systems.
Moderna’s vaccine appears to cause these side effects more often than some other standard vaccines, but the reactions still seem to be relatively moderate and temporary, according to Benkeser.
On Friday, Marks, the FDA official, said that he wasn’t concerned that these side effects would discourage people from getting a shot. “I think that ultimately if these are managed well,” he said, “if we communicate the expectations well, I think particularly among those who are eager to get back to a normal life — which I think that includes many of us — there will be reasonable uptake of this and we’ll continue to communicate about these side effects to help people be prepared to know what to expect.”
And the rate of serious side effects was low — 1% — and about the same in both the vaccine and placebo groups, leading the FDA to conclude that there wasn’t clear evidence that it was the vaccine that caused them.
The most serious side effect reported was Bell’s palsy, a temporary drooping of facial muscles, seen in three members of the vaccine group and one placebo group member. (Bell’s palsy was also seen in four people who received the Pfizer vaccine.) FDA scientists compared the incidence of Bell’s palsy to how frequently it appears in the general population and have concluded that there is no evidence they are linked to the vaccine. Advisory panel member Paul Offit of the Children’s Hospital of Philadelphia questioned the agency’s suggestion that the palsy reports were flukes, noting that COVID-19 also reportedly causes the temporary drooping. The agency will continue to monitor for further cases.
Moderna’s Tal Zaks said the company would watch for any more cases among people who receive the vaccine after authorization, in conjunction with federal safety monitoring systems.
Another reported side effect that’s perhaps noteworthy for those who have had lip injections in the past: A 29-year-old woman in the vaccine group who had undergone the cosmetic procedures had her lips swell up after getting her shots. Two other participants with a history of “cosmetic filler injections'' experienced facial swelling too. The FDA speculated that this could be due to an inflammatory reaction between the immune response prompted by the vaccination and the filler substance. FDA staff said Thursday that the swelling incidents resolved quickly with steroids, and that this possible side effect would be noted in the prescribing information.
Other questions have been raised about whether it is safe for people with severe allergies to get vaccinated with Pfizer’s vaccine, and if those concerns might apply to Moderna’s vaccine. This week, there were reports of two people at an Alaska hospital who experienced allergic reactions after getting Pfizer’s immunization. On Thursday, FDA staff said the agency did not have enough information about the cases, but was investigating alongside the CDC. FDA officials also said that in Moderna’s clinical trial, there were reports on rashes, hives, and itching, but “nothing that really are close even to anaphylaxis,” or a severe, potentially life-threatening allergic reaction.
In the US, the prescribing information for Moderna’s vaccine — as with Pfizer’s — says that it should be avoided by people with a history of severe allergies to any component of the vaccine.
On Friday, Marks said that one ingredient in both vaccines, polyethylene glycol, can be associated with allergic reactions in general and “could be a culprit here.” But he stressed that was for now a matter of speculation and that the agency would be closely watching the rollout of the Moderna vaccine. “We don’t know that for a fact,” he said. “We just don’t know at this point.”
As of Friday evening, the FDA was aware of “roughly about five” allergic reactions in possible connection with the Pfizer vaccine in different states, including Alaska, according to Marks. But “it’s difficult to talk about them with any kind of good certainty until we have more information about them,” he said.
4. The Moderna vaccine has some clear advantages over Pfizer’s.
Moderna’s vaccine looks like it has two advantages over Pfizer’s: It may be better at preventing severe cases and will be far easier to distribute nationwide.
Data indicated that Moderna’s vaccine could prevent illnesses that required oxygen or hospitalization — the kinds of cases that are overwhelming intensive care units around the country. While 30 people in the placebo group got seriously ill starting two weeks after their second dose, none of the vaccinated people did. Pfizer’s trial, in contrast, produced less evidence showing it could stave off these severe cases.
“It’s not to say that Moderna’s vaccine is necessarily better at preventing severe disease than Pfizer’s, just that we’re more confident in the results that we’ve seen from Moderna at this point,” Benkeser said.
The two vaccines were tested on slightly different age ranges of people: Moderna’s in adults 18 and older, Pfizer’s in people as young as 12 (and authorized for ages 16 and up). The efficacy of the Moderna vaccine in reducing COVID-19 diagnoses was also slightly lower in people 65 and older, but still high, at 86.4%, compared to Pfizer’s 93.7%.
Moderna’s vaccine also has some advantages in terms of how easily it can be delivered to people. The Moderna vaccine should be much easier to administer to people outside of massive medical facilities and large cities. It is shipped at freezer temperatures of -4 degrees Fahrenheit, making it a lot easier to manage than the Pfizer one, which requires dry ice shipping and storage at the ultracold temperature of -94 degrees. Moderna’s vials can be stored at refrigerator temperatures for up to 30 days, and at room temperatures for half a day.
It also comes in smaller 100-dose packages, unlike Pfizer’s dry ice boxes, which contain 975 doses that need to be used quickly for large numbers of volunteers to prevent spoilage. Pfizer’s large packages have made the vaccine inaccessible in less populated rural areas that can’t use up the full supply of doses.
In the coming weeks, authorization of Moderna’s vaccine will allow more doses to be sent to rural areas and nursing homes, OWS’s Gen. Gus Perna said on Wednesday. Some 3,285 sites are already planned for its distribution, and discussions are underway with 19 pharmacy chains for roughly 7,000 in-store immunization sites. In comparison, the Pfizer vaccine was shipped to just 636 sites this week.

5. The results are good news for mRNA vaccines.
Both the Moderna and Pfizer vaccines are mRNA vaccines, which inject genes for the spike protein of the coronavirus into the body, producing an immune reaction that guards against the virus. This is a new approach to vaccines, with the Pfizer authorization a first of its kind.
It’s not a coincidence that these vaccines were first to the finish line. Traditional vaccines need to grow in eggs or cells before they are inactivated and injected into people. But with mRNA vaccines, all you need to start one is the genetic code for the virus you are aiming for.
“I just want to make the point that what a remarkable scientific achievement this is and say thanks to all the scientists present and past who contributed to this,” James Hildreth, a professor of medicine at Meharry Medical College, said at Thursday’s panel meeting. “To go from having a sequence of a virus in January to having two vaccines available in December is a remarkable achievement.”
Although investigational mRNA vaccines for rabies, Zika, and other viruses have been developed, none had yet been authorized for use outside of clinical trials.
The success of the COVID-19 vaccines bodes well for this kind of vaccine in attacking not just coronaviruses but others such as flu or meningococcal viruses as well, Biotechnology Innovation Organization Chair Jeremy Levin told BuzzFeed News. Vaccine experts envision an era of “vaccines on demand,” where new immunizations can be created regularly against newly emerging diseases.
“We can expect to see this kind of rapid development looking ahead,” Levin said. One added advantage of mRNA vaccines is in responding to viruses that rapidly mutate, like the flu, which every year requires new vaccines targeted to their most common strains. In the event that SARS-CoV-2 mutates in response to immunization by humanity in the next few years, mRNA vaccines could be very useful.
6. Taxpayers bankrolled the Moderna vaccine from start to finish.
One final, noteworthy, difference between Pfizer’s and Moderna’s vaccines that OWS chief scientist Moncef Slaoui has noted is that the latter was designed, tested, and manufactured in close consultation with federal officials. (Pfizer’s partnership with the government only extends to purchasing doses.) That gives OWS much more insight into how reliable Moderna’s manufacturing projections are. The US has already contracted for another 100 million doses of its vaccine in the spring.
Negotiations are still ongoing for 100 million more doses from Pfizer, which has been troubled by raw material delays in its vaccine manufacturing. On Wednesday, Slaoui said OWS had only recently learned about the manufacturing problems. (Pfizer on Thursday issued a statement saying its manufacturing plan was on track.)
Scientists at the National Institutes of Health worked hand in hand with Moderna to both design its vaccine and start initial testing in people. The vaccine was designed in January within days of the virus’s genome being released from China, at the behest of National Institute of Allergy and Infectious Diseases chief Anthony Fauci. As part of the $14 billion OWS, taxpayers have bankrolled the Moderna vaccine from start to finish.
Fundamentally, both vaccines were designed based on the NIH-funded method for freezing the spike of a coronavirus in the shape it takes prior to infection, research that grew out of the 2004 SARS coronavirus outbreak.
“Operation Warp Speed is built on decades of government-funded research,” said Zain Rizvi of the watchdog group Public Citizen.
UPDATE
This story has been updated to state that the FDA authorized the vaccine on Friday.
CORRECTION
A previous version of this post misstated that the Moderna vaccine's first dose offers better protection than Pfizer's.
UPDATE
This story has been updated to clarify what Moderna’s clinical trial data suggests about the vaccine’s ability to prevent severe illness.

